Somos® BioClear™
Print clear, accurate guides and models for peace of mind
Somos® BioClear is specially developed for use in medical applications, meeting the requirements for non-implantable limited body contact (<24hr) medical and dental applications. Somos® BioClear has passed stringent ISO 10993-5 Cytotoxicity, ISO 10993-10 Irritation & Sensitization and USP Class VI testing, after following the cleaning procedure as described in the Somos® BioClear user guide.
Parts produced from Somos® BioClear are clear and have ABS-like mechanical properties and a good combination of strength and toughness. The material is very resistant to moisture and many common solvents and chemicals.
Key Features
- Passed stringent ISO 10993-5 Cytotoxicity, ISO 10993-10 Irritation & Sensitization and USP Class VI testing
- High accuracy and surface quality
- High moisture resistance
- Complex shapes and feature detail
- Eliminates chemical texturing process
- Exceptional clarity facilitates inspection of feature detail and quality
- Resistant to common solvents
Applications
- Prototyping
- Tooling
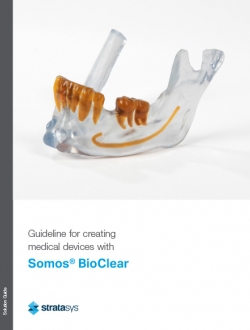
- Surgical guides
- Anatomical models for surgical planning
- Non-implantable/limited contact medical applications
- Functional prototypes with body contact
Resources:
Manufacturer: