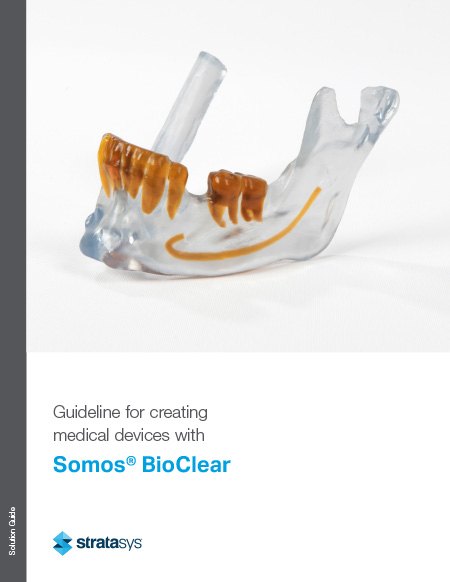
Creating Medical Devices with Somos Bioclear
Somos® BioClear has passed cytotoxicity, irritation, sensitization, and USP VI. A toxicological profiling of the components has been performed. A Device Master File to support this material is accessible through the FDA. Ask a sales representative for more information.
Steps in the production process of 3D printed parts can influence the final part safety. This document gives guidance on areas to pay attention to when using Somos® BioClear.